
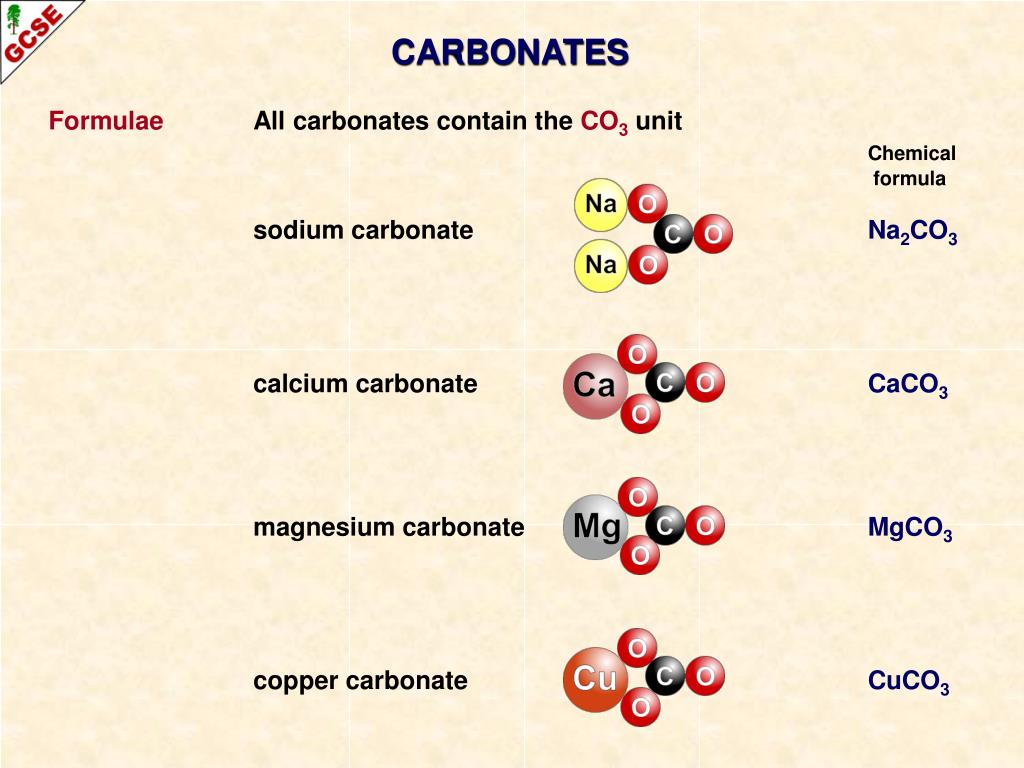
In conclusion, the Lewis structure of the carbonate ion is an important tool in understanding the chemical properties of the molecule. This information can be used to predict the behavior of carbonate ion in chemical reactions and its interactions with other molecules. For example, it shows that the ion has a trigonal planar geometry, and that the oxygen atoms are negatively charged, while the carbon atom is positively charged. The Lewis structure of the carbonate ion provides important information about the chemical properties of the molecule. The carbon, in the carbonate ion, has 4 x 1 4 electrons assigned to it (one from each of its four bonds), therefore it has a formal charge of zero (neutral). The negative charge on the ion is located on two of the oxygen atoms, to indicate that it has gained two electrons and achieved a stable octet configuration. The remaining electrons are distributed as lone pairs on the oxygen atoms. In this structure, the carbon atom is connected to three oxygen atoms, with one double bond and two single bonds. The final Lewis structure of CO 3 2- can be represented as: Lewis structure of carbonate ion CO 3 2. This will give the carbon atom a complete octet of electrons, and each oxygen atom will still have a complete octet of electrons. In calcite, one carbon and three oxygen atoms are held together by covalent bonds to form a molecular ion, called carbonate, which has a negative charge. To complete the octet of the carbon atom, we can use one of the lone pairs on one of the oxygen atoms to form a double bond with carbon. This arrangement gives each oxygen atom a complete octet of electrons, while the carbon atom has only six electrons. Therefore, we can place a double bond between the carbon atom and one of the oxygen atoms, and single bonds between the carbon atom and the remaining two oxygen atoms. Carbon can form four bonds, while oxygen can form two bonds. Next, we need to connect the atoms in the molecule using single or double bonds.
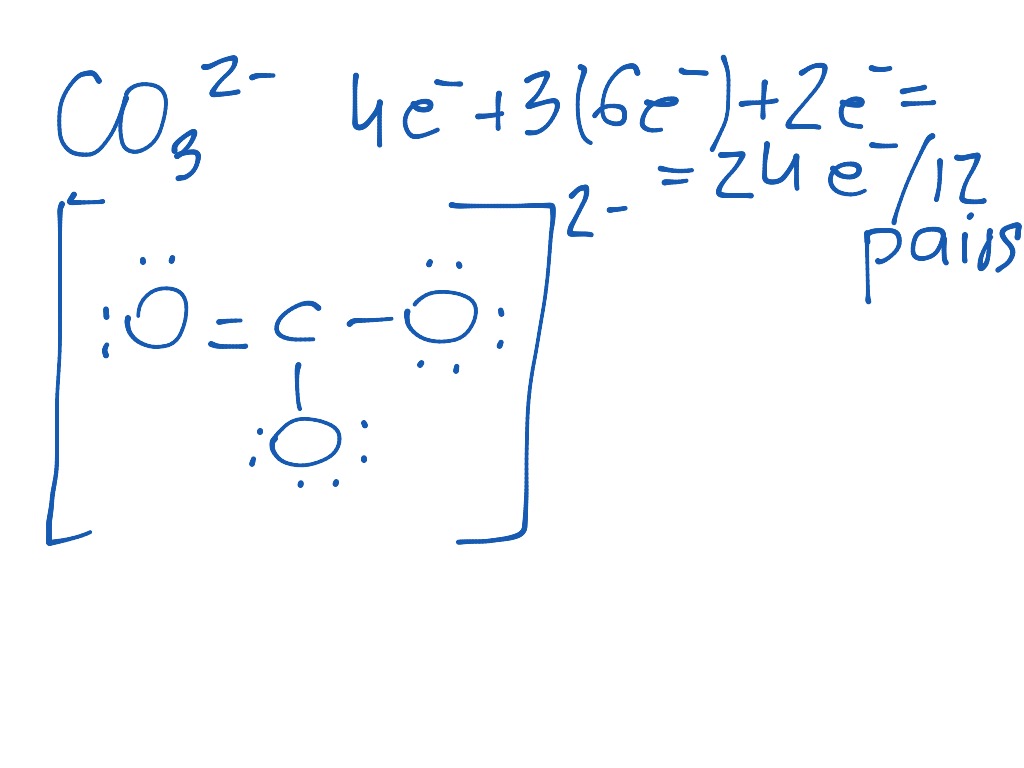
Hence, a pair of electrons from oxygen needs to come over and form a double bond.
Charge of carbon in co3 full#
However, note that carbon does not have a full octet shell. Hence, there are 24 electrons in total ( 4 +18 +2 24) Carbon would be in the middle which gives you. The extra two electrons are added to the total because the ion carries a -2 charge. And the charge of 2 gives you an additional 2 electrons. Therefore, the total number of valence electrons in CO 3 2- is: Carbon has four valence electrons, while each oxygen atom has six valence electrons. In CO 3 2-, we have one carbon atom and three oxygen atoms. The formal charge on three oxygen atom has electron pair shared by chlorine. Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. Click hereto get an answer to your question Calculate formal charge of atoms HClO4, CO32. To draw the Lewis structure of CO 3 2-, we first need to determine the total number of valence electrons present in the molecule. What is the Lewis structure of carbonate ion CO 3 2-?


 0 kommentar(er)
0 kommentar(er)
